Química de Productos Naturales Marinos
Los organismos marinos constituyen una importante fuente de productos naturales con características estructurales singulares y con un enorme potencial para su aplicación en la industria biomédica, agroquímica y cosmética.
Perfil del grupo Química de Productos Naturales Marinos en Digital.CSIC.

Presentación
La investigación que se desarrolla en el grupo de Química de Productos Naturales Marinos del IPNA tiene como principal objetivo el descubrimiento de nuevas moléculas de macroorganismos y microorganismos marinos y estudiar su potencial terapéutico.
Líneas de investigación

Determinación de la actividad biológica de extractos y de compuestos puros
El potencial terapéutico e interés industrial de los extractos y productos puros se establece a través de los ensayos de actividad antibacteriana y antifúngica que realizamos en el laboratorio y mediante colaboraciones con otros grupos de investigación y con empresas.
Microorganismos marinos
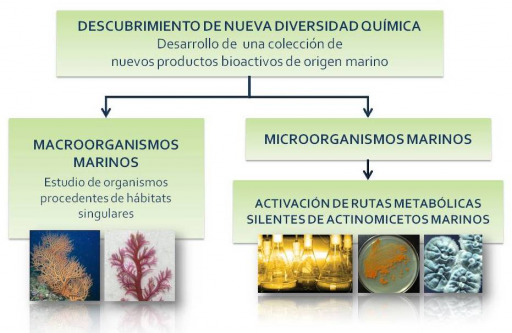
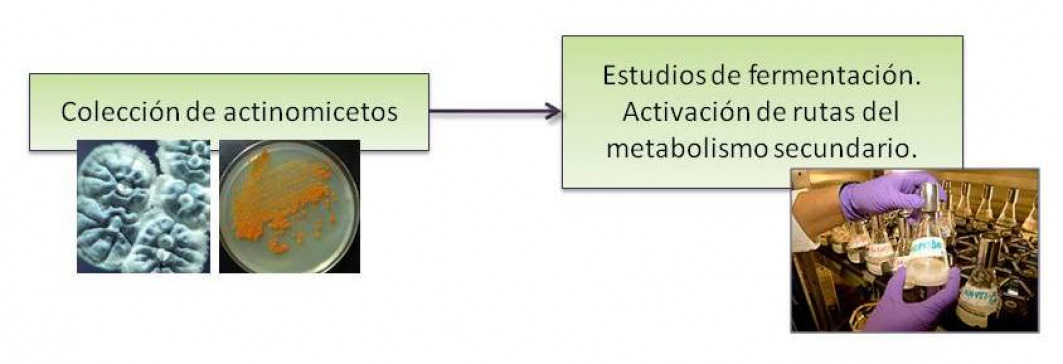
Los microorganismos marinos han desarrollado mecanismos fisiológicos y bioquímicos únicos para sobrevivir en hábitats extremos y altamente competitivos produciendo metabolitos secundarios únicos y con alto valor terapéutico. Otra de nuestras líneas de investigación consiste en el estudio...
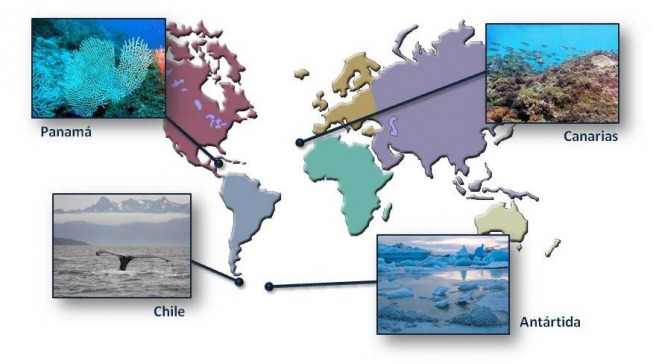
Macroorganismos marinos
La producción de metabolitos secundarios es dependiente del hábitat, por lo que una parte de nuestro trabajo se centra en el estudio de algas, esponjas, moluscos, corales,… procedentes de diversos hábitats tales como: Panamá, Chile, Antártida y Canarias.
Formación
Sustancias bioactivas de algas e invertebrados marinos de hábitats diversos: Ptilonia magellanica, Aplysia dactylomela y Leptogorgia sp.
Estudio Químico y Evaluación Biológica de metabolitos secundarios de octocorales
Estudio Químico de Invertebrado Marinos y Microorganismos Asociados
Terpenoides Bioactivos y Acetogeninas de Algas e Invertebrados Marinos
Metabolitos producidos por el alga roja Ptilonia magellanica. Estructura y citotoxicidad
Metabolitos producidos por un hongo de origen marino. Estructura y citotoxicidad
Nuevos Cembranoides del octocoral Leptogorgia sp. Como Posibles Marcadores Quicio-taxonómicos
Biodiversidad de Algas y Esponjas Marinas como Fuente de Nuevas Sustancias Bioactivas
Financiación

Islas Canarias: Naturaleza Marina Singular, Salud y Bienestar
A través de la biotecnología azul, este proyecto pretende poner en valor la singularidad de la biodiversidad microbiológica del medio marino de Canarias, influenciado por sus volcanes, como fuente...
En Ejecución

Bacterias marinas para mejorar la calidad y la seguridad alimentaria
En Ejecución

Metabolímica y quimiometría: herramientas eficientes y eficaces para el estudio biotecnológico de la diversidad química de productos naturales de la microbiota marina
NatháLia Peixoto Nocchi Carneiro
En Ejecución

Microbiota de hábitats marinos para el desarrollo de agentes terapéuticos y compuestos de alto valor añadido
En Ejecución

EU COST Action CA18238 - A transdisciplinary network for establishing a marine biotechnology community
Susana P. Gaudencio (WG2) y Arita Dubnika (WG3)
En Ejecución

Bioprospección de bacterias marinas del volcán Cumbre Vieja para el desarrollo de nuevos agentes terapéuticos
Sara García Davis
En Ejecución

Biotecnología de microalgas: Desarrollo del potencial biotecnológico de cepas del dinoflagelado del género Amphidinium de las Islas Canarias
M. Luisa Souto Suárez
En Ejecución

Moléculas bioactivas de microalgas marinas
José J. Fernández
En Ejecución

Bacterias obligadamente marinas como productores de moléculas con potencial antimicrobiano (antimicrobiano y antifúngico) para su aplicación en el sector agroalimentario (RTA 2015-00010-C03-02)
Finalizado

Microorganismos marinos como productores de moléculas con potencial terapéutico
Finalizado

Productos naturales de organismos marinos antárticos contra la proliferación celular
Finalizado

Microorganismos marinos como productores de moléculas con potencial terapéutico en respuesta a un proceso de inducción mediante co-cultivo de diferentes especies
Finalizado

La biodiversidad de las islas Canarias como fuente de nuevas moléculas bioactivas de interés farmacéutico
Finalizado

La Biodiversidad en Ecosistemas Marinos de las Islas Occidentales de La Palma y el Hierro como Fuente de Nuevas Moléculas Bioactivas de Interés Farmacológico
Finalizado
Personal
Mercedes Cueto Prieto
Publicaciones
Chloro-Furanocembranolides from Leptogorgia sp. Improve Pancreatic Beta-Cell Proliferation
Two new chloro-furanocembranolides (1, 2) and two new 1,4-diketo cembranolides (3, 4) were isolated from the crude extract of Leptogorgia sp. together with a new seco-furanocembranolide (5) and the known Z-deoxypukalide (6), rubifolide (7), scabrolide D (8) and epoxylophodione (9). Their structures were determined based on spectroscopic evidence. Four compounds: 1, 2, 7 and 8 were found to activate the proliferation of pancreatic insulin-producing (beta) cells.
Gallardo, Amalia; Díaz-Marrero, Ana R. ; Rosa, José M. de la ; D'Croz, Luis; Perdomo, Germán; Cózar-Castellano, Irene; Darias, Jose; Cueto, Mercedes
A set of biogenetically interesting polyhalogenated acetogenins from Ptilonia magellanica
Ptilonines A−F, pyranosylmagellanicus D−E and magellenediol are previously undescribed acetogenins isolated from the red alga Ptilonia magellanica. Their structures were determined from spectroscopic evidence. The absolute configuration of the known pyranosylmagellanicus A, was established by derivatization with (R)− and (S)−α−methoxy −α−phenylacetic acids (MPA). Ptilonines exhibit an unusual halogenation pattern, that may confer evolutionary advantages to Ptilonia magellanica, for which a biogenetic origin is proposed. The antimicrobial effect of some of these compounds was evaluated.
Gallardo, Amalia; Cueto, Mercedes; Díaz-Marrero, Ana R. ; Rosa, José M. de la; Fajardo, Víctor; San-Martín, Aurelio,; Darias, José
Leptolide Improves Insulin Resistance in Diet-Induced Obese Mice
Type 2 diabetes (T2DM) is a complex disease linked to pancreatic beta-cell failure and insulin resistance. Current antidiabetic treatment regimens for T2DM include insulin sensitizers and insulin secretagogues. We have previously demonstrated that leptolide, a member of the furanocembranolides family, promotes pancreatic beta-cell proliferation in mice. Considering the beneficial effects of leptolide in diabetic mice, in this study, we aimed to address the capability of leptolide to improve insulin resistance associated with the pathology of obesity. To this end, we tested the hypothesis that leptolide should protect against fatty acid-induced insulin resistance in hepatocytes. In a time-dependent manner, leptolide (0.1 µM) augmented insulin-stimulated phosphorylation of protein kinase B (PKB) by two-fold above vehicle-treated HepG2 cells. In addition, leptolide (0.1 µM) counteracted palmitate-induced insulin resistance by augmenting by four-fold insulin-stimulated phosphorylation of PKB in HepG2 cells. In vivo, acute intraperitoneal administration of leptolide (0.1 mg/kg and 1 mg/kg) improved glucose tolerance and insulin sensitivity in lean mice. Likewise, prolonged leptolide treatment (0.1 mg/kg) in diet-induced obese mice improved insulin sensitivity. These effects were paralleled with an ~50% increased of insulin-stimulated phosphorylation of PKB in liver and skeletal muscle and reduced circulating pro-inflammatory cytokines in obese mice. We concluded that leptolide significantly improves insulin sensitivity in vitro and in obese mice, suggesting that leptolide may be another potential treatment for T2DM
Villa-Pérez, Pablo; Cueto, Mercedes; Díaz-Marrero, Ana R; Lobatón, Carmen D.; Moreno, Alfredo; Perdomo, Germán; Cózar-Castellano, Irene
The polyphenol altenusin inhibits in vitro fibrillization of tau and reduces induced tau pathology in primary neurons
In Alzheimer’s disease, the microtubule-associated protein tau forms intracellular neurofibrillary tangles (NFTs). A critical step in the formation of NFTs is the conversion of soluble tau into insoluble filaments. Accordingly, a current therapeutic strategy in clinical trials is aimed at preventing tau aggregation. Here, we assessed altenusin, a bioactive polyphenolic compound, for its potential to inhibit tau aggregation. Altenusin inhibits aggregation of tau protein into paired helical filaments in vitro. This was associated with stabilization of tau dimers and other oligomers into globular structures as revealed by atomic force microscopy. Moreover, altenusin reduced tau phosphorylation in cells expressing pathogenic tau, and prevented neuritic tau pathology induced by incubation of primary neurons with tau fibrils. However, treatment of tau transgenic mice did not improve neuropathology and functional deficits. Taken together, altenusin prevents tau fibrillization in vitro and induced tau pathology in neurons.
Chua, Sook Wern; Cornejo, Alberto; Eersel, Janet van; Stevens, Claire H.; Vaca, Inmaculada; Cueto, Mercedes; Kassiou, Michael; Gladbach, Amadeus; Macmillan, Alex; Lewis, Lev; Whan, Renee; Ittner, Lars M.
Oxysterols from an octocoral of the genus Gorgonia from the eastern Pacific of Panama
Eighteen new oxysterols have been isolated from a previously undescribed octocoral collected from the eastern Pacific of Panama. Their structures were determined based on spectroscopic evidence. The absolute configuration was established by derivatization with (R)- and (S)-MPA. Antimicrobial and antileishmanial effects were evaluated.
Cardoso-Martínez, F.; Rosa, José M. de la ; Díaz-Marrero, Ana R.; Darias, José; D'Croz, Luis; Jiménez-Antón, M. Dolores; Corral, María Jesús; García, Rocío; Alunda Rodríguez, Jose María; Cueto, Mercedes
Tanzawaic acids isolated from a marine-derived fungus of the genus Penicillium with cytotoxic activities
Tanzawaic acids M (1), N (2), O (3) and P (4) and the known tanzawaic acids B (5) and E (6), have been isolated from an extract of a cultured marine-derived fungus (strain CF07370) identified as a member of the genus Penicillium. The structures of 1–4 were determined based on spectroscopic evidence. The antimicrobial and cytotoxic activities of compounds 1–6 were evaluated.
Cardoso-Martínez, F.; Rosa, José M. de la; Díaz-Marrero, Ana R.; Darias, José; Cerella, Claudia; Diederich, Marc; Cueto, Mercedes
Mercedes Cueto Prieto

Datos de contacto
Otros grupos de investigación