Oxidative and photoredox decarboxylation of cyclodextrins
In our group, we have experience in functionalization of uronic acids,1 and α-amino acids2 by decarboxylation, in an oxidizing medium, with the use of PhI(OAc)2 and iodine. The methodology we use involves mild reaction conditions, easy handling and the use of commercial or readily accessible oxidants, non-toxic or polluting. However, until now we have not extended this protocol to the generation of 5-pentapyranosyl radicals in more complex carbohydrate systems such as disaccharides or cyclic polysaccharides like cyclodextrins (CDs). This is despite the fact that they offer the possibility of carrying out functionalizations of interest that can affect, in the case of CDs especially, their physical-chemical qualities, and therefore their future applications.
The ability of CDs to encapsulate biomolecules in their internal cavity is well known, and their potential application as nanocarriers has stimulated intense research to modify and improve their properties. However, curiously, among all the methodologies described to carry out these modifications, radical reactions in CDs are practically unknown.
Credits: J. Agric. Food Chem. 2013, 61, 34, 8156–8165
In this line of research, we would like to generalize our protocol for oxidative β-fragmentation (decarboxylation) applying it to carboxylic acids in position 5 of α-maltose systems, a structural subunit of CDs, and then extending the methodology to mono- and di-acids of α- and β-CDs.
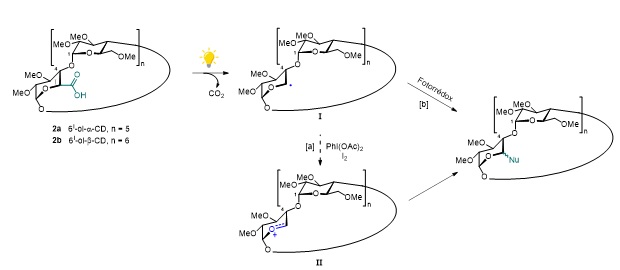
With a comparative purpose and with the idea of obtaining a more extensive series of functionalized CDs, we also will carry out photoinduced radical decarboxylation by testing different photoredox catalysts and carbon nucleophiles using recent methodologies described in the literature.
With these studies, we will acquire knowledge for basic synthetic chemistry but a library of modified CDs will be created as well, which will be subjected to activity studies to analyze their possible applications.
1) Tetrahedron Lett. 1997, 38, 4141–4144.
2) a) J. Org. Chem. 2000, 65, 4930-4937. b) Tetrahedron Lett. 1999, 40, 5945–5948.
Inés Pérez Martín
