Molecular recognition and organocatalysis: mimicking enzymes
Research lines:
Chiral Cation Receptors
Non-covalent interactions are responsible for a large number of the functions of biological systems: molecular recognition, transport, catalysis, etc. Therefore, understanding these interactions is essential for modulating the functional properties of molecules and new materials. We have used chiral cation receptors as simple models to be able to quantify some of these interactions and the way in which they cooperate with each other.
Peptide-Mimetic Synthesis and Organocatalysis
Another model we use to study non-covalent interactions are mimetic peptides. These structures are used to mimic the peptide structure by spatially arranging functional groups in a controlled way. Possessing characteristics similar to their natural counterparts, mimetic peptides have been used to replace enzymes or proteins, or to interrupt interactions between proteins. Our group uses sugar-amino acids (SAA) as a unit for the construction of peptide-mimetics, which possess the skeleton of a sugar, and additionally an amino and an acid functionality. Studying cyclic peptides derived from ε-SAA, we found that small modifications in the skeleton of ε-SAA can lead to changes in the pattern of intramolecular non-covalent interactions and therefore significantly modify the spatial arrangement of these macrocycles. Transferring this knowledge to organocatalysis we have designed two pseudoenantiomeric catalysts capable of catalyzing the Michael addition of aldehydes to β-nitrosostyrenes. The reactions proceed in an enantioselective way using catalytic loads minor to 1 mol %, in practically quantitative yields and with enantiomeric excesses higher than 97 %.
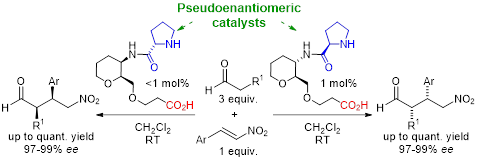
We are currently working to expand the use of these highly modulable organocatalysts to new reactions and substrates.
Tomás Martín Ruíz
