Simple and direct synthesis of 2-aminopyrroles
Domino is the game: The development of synthetic methodologies designed to mimic Nature’s efficiency when constructing molecular architectures remains as one the most difficult tasks for synthetic chemists. An approach to this challenge relies on the development of “continuous” molecular construction processes, i.e. processes performed in one synthetic step and involving more than one synthetic transformation. A practical manner to perform these processes is through the so-called domino effect, i.e. the cumulative effect that results when one event triggers a series of consecutive events. In the laboratory, these processes are hosted by molecular platforms endowed with a set of compatible organic functionalities, which must be capable of being activated in a sequential and orderly manner by the action of an external stimulus (heat, light, chemical trigger). Once the stimulus is applied, an ordered sequence of chained reactions takes place following the specific construction code designed for the assembly of the target molecular structure from this particular array of chemical functionalities.
This mode of molecular construction has practical advantages in terms of both its instrumental simplicity and labor and resources economy, but it requires a careful and complex design because only one of the many possible molecular structures should be produced from the combination of functions present in the platform. The generation and control of reactivity are the keys to orchestrate these processes selectively and efficiently. In this line, the research team led by Dr. García Tellado y Dr. Tejedor Aragón from the Structure, Design and Molecular Function Group of the Instituto de Productos Naturales y Agrobiología (CSIC), has developed a simple and direct methodology to construct 2-aminiopyrroles, a common structural motive to a large number of therapeutically important molecules. The methodology uses N-alkynyl-N’-vinylhydrazides as molecular platforms (see figure for structure), heat as external stimulus, and a novel molecular rearrangement as the domino process trigger.
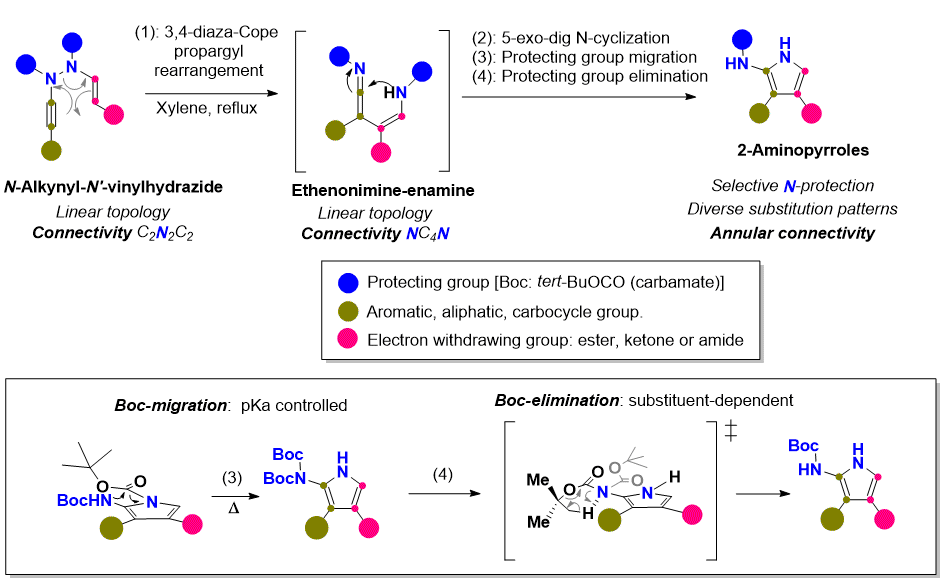
This work has been recently published in Organic Letters and it is part of the thesis work of Raquel Diana-Rivero.
Raquel Diana-Rivero, Beate Halsvik, Fernando García-Tellado, David Tejedor. Short and Modular Synthesis of Substituted 2‑Aminopyrroles. Org. Lett. 2021, 23, 4078-4082. DOI: 10.1021/acs.orglett.1c01345.
