GeoBiomimetic Applications of the Iodine Reactivity in the Field of the Organic Synthesis
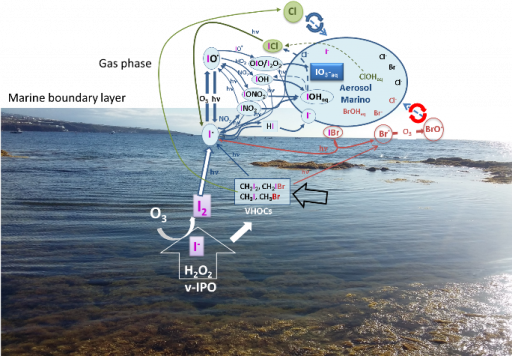
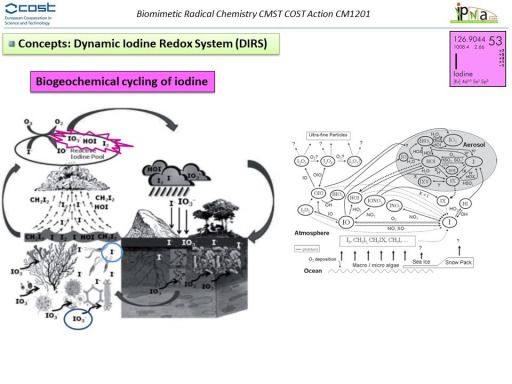
Iodine is a scarce element in Earth (13.6 ppb by weight), occurring mainly in its three more stable oxidation states: iodide (-1), iodate (+5) and periodate (+7). However, iodine chemistry is surprisingly highly relevant in fundamental biological, geophysical, atmospheric or chemical processes and even more remarkable is the fact that the highly unstable oxidation states [I(0), I(+1) and I(+3)] are the responsible for these affair despite its non-significant presence in nature. That incidence could be explained considering that iodine is mainly contributing to many of these natural processes in a catalytic manner rather than in stoichiometric way. Accordingly, it is also noteworthy the few examples of iodine-containing natural products documented to date, among which, small iodoalkanes and polyhaloalkanes that belong to the group of volatile halogenated organic compounds (VHOCs) globally predominate. These VHOCs are released by marine organisms to the atmosphere and, in turn, function as iodine carriers and recyclers (transient species).
Also, iodine is considered as an essential trace element for life in higher animals and especially relevant in humans, where its deficiency usually leads to biological disorders.
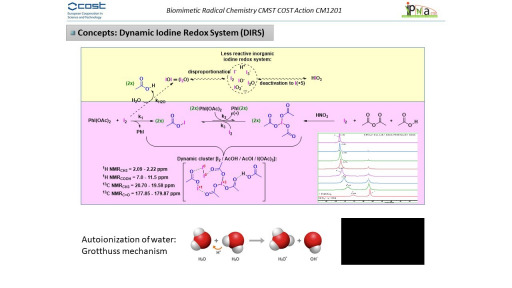
In general, and under a biological and atmospheric point of view, the iodine redox system can be considered as a “catalytic antioxidant” that requires little doses of iodine to reduce large amounts of oxidants such as H2O2 or ozone.
In Organic Chemistry, the easy conversions between the different oxidation states of iodine has served to develop a wide number of chemical transformation of organic matter where iodine is not incorporated into the final products, and among them oxidative processes predominate. Thus, many novel methodologies based in iodine-catalysis, such as functional groups oxidations or oxidative coupling reactions, has been reported in literature, prompted by the recent advances in the determination of the role of iodine chemistry in the reaction mechanisms. Impressive is the current advances in new iodine-catalyzed oxidative coupling reactions as greener alternative to the transition-metal catalysis, despite the exact role of iodine catalysis is still rather limited.
In our research line, our efforts are focused on a better understanding on the fascinating redox iodine systems in different environments. A relevant observed feature of this redox system has been the dynamicity within the iodine transient species interconversions driving us to the concept of Dynamic Iodine Redox Systems (DIRS).
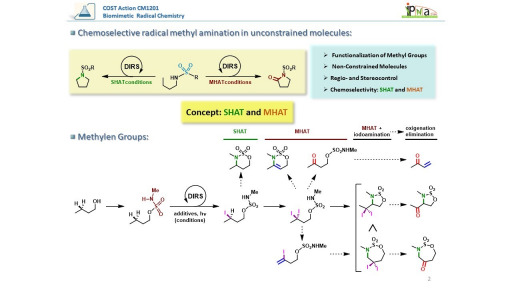
As a consequence, the modulation of the DIRS has enabled the development of different approaches to achieve the chemoselective functionalization of various C–H bonds employing sulphonamides as directing groups, enabling the establishment of the concept “SHAT-MHAT-OX” (simple Hydrogen Atom Transfer-Multiple Hydrogen Atom Transfer-Oxidation).
Antonio Jesús Herrera González