Structure, design and molecular function
Presentation
We develop research programs aimed to the:
- Development of new sustainable and simple synthetic methodologies for the generation of new chemotypes, and their rational transformation into high-value molecules.
- Development of new methodologies for the structural determination of organic molecules.
- Inquire on fundamental questions of supramolecular chemistry such as the nature of non-covalent interactions or ligand-biomolecule interactions.
- Design of new stimuli-responding macromolecular devices capable of carrying out a function.
Research lines
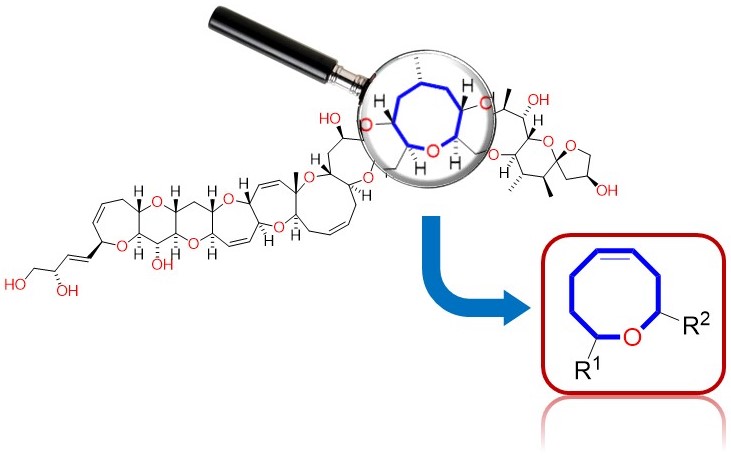
Molecular structure and function: spectroscopic and computacional methods
Sustainable metal catalysis. Application in heterocyclic chemistry, and biologically active natural products and derivatives
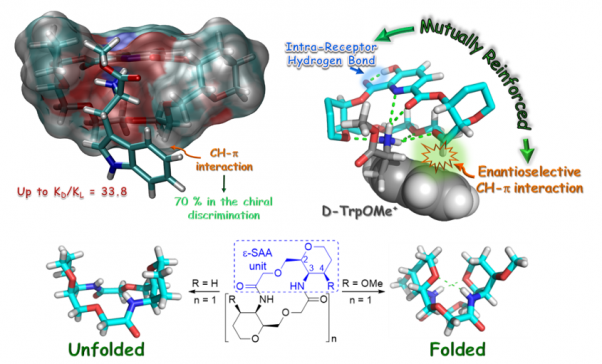
Molecular recognition and organocatalysis: mimicking enzymes
Our objective is to design and synthesize simple molecular models that allow us to study and quantify non-covalent interactions, extrapolating these interactions to the development of new and more efficient organocatalysts.
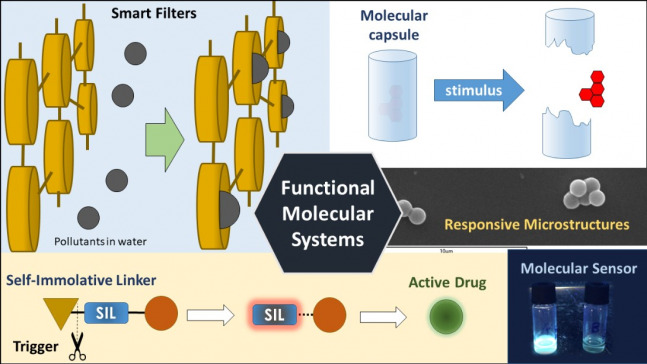
Functional Molecular Systems
Our main aim is to design and construct molecular or supramolecular systems that are able to perform a task. We are particularly interested in those molecules or materials which are able to respond to a stimulus, which triggers a cascade of events, ending up with the desired function.
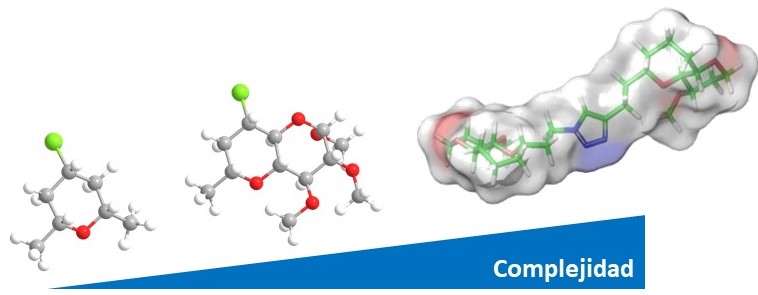
Molecular complexity and function
We are active in the organic synthesis field. Our main ethos is the design and development of novel synthetic methods for sustainable and efficient molecular construction. Our methodologies are based on novel chemical reactivity models, organocatalysis and domino processes (cascade).

Medicinal chemistry. Natural products inspired molecular design
The main interest of this research group is the design and synthesis of new molecules that can display relevant bioactivities interacting with biological systems. The development of these compounds is carried out through simple strategies and taking advantage of structures inspired by natural...
PhD & MSc. Thesis
TFM: Self-Immolative Molecular Capsules
TFM: Sensores moleculares fluorescentes auto-inmolativos
TFM: Pro-fármacos antitumorales inspirados en el ciclo redox de la menadiona promovida por el ascorbato
TFG: Síntesis de derivados de ascorbato y menadiona como potenciales antitumorales
Síntesis de Productos de Alto Valor Añadido a Través de Metodologías Sintéticas de Baja Toxicidad
Nueva Metodología para la Construcción Molecular Modular y Orientada a la Diversidad
1,4-Diinos terciarios activados. Nuevas plataformas para la generación de complejidad y diversidad estructural
Síntesis Estereoselectiva de Moléculas Bioactivas a Partir de Carbohidratos
Nuevas Metodologías Sintéticas Orientadas a la Diversidad
Master en Biomedicina, Especialidad en Diseño y Desarrollo Preclínico de Fármacos
1-4-DIÍNOS ACTIVADOS: PLATAFORMAS PARA LA GENERACIÓN DE DIVERSIDAD
Master de Investigación en Química. Intensificación Química Orgánica
Nuevas Metodologías Sintéticas Orientadas a la Diversidad
Éteres propargílicos vinílicos: plataformas para la generación de diversidad estructural
Reconocimiento Molecular: Diseño y Síntesis de una Nueva Familia de Módulos Estructurales. Síntesis Radicalaria de Carbociclos en Medios no Reductivos. Síntesis Formal de la (+)-Preusina: Un Nuevo Acceso A Pirrolidinas Quirales
Reacciones Multicomponente en Agua
Máster de Investigación en Química. Intensificación Química Orgánica
Propargyl Vinyl Ethers: Synthetic Applications
Aplicación de la Reacción de Nicholas en la Síntesis de Éteres Cíclicos
Diseño, Síntesis y Estudio de Nuevos Receptores Quirales de Cationes (DEA)
Nuevos Receptores Quirales de Cationes: Diseño y Síntesis (TFM)
Reacción de Nicholas Intramolecular Estereoselectiva empleando Epóxidos como Nucleófilos (DEA)
Del Reconocimiento Molecular a la Organocatálisis: La unidad de Tetrahidropirano como Motivo Estructural Privilegiado
Síntesis de Éteres Cíclicos a Través de Ciclaciones en Cascada de Epóxidos (DEA)
Receptores Quirales de Cationes y Foldámeros como Modelos para el Estudio del Plegamiento Molecular y sus Consecuencias
Diseño y Síntesis de Nuevas Unidades de Aminoácidos no Naturales con Aplicación en la Formación de Péptidos Miméticos (DEA)
Uso de la Reacción de Nicholas Intermolecular y Metátesis de Cierre de Anillos en la Formación de Éteres Cíclicos con Alto Grado de Sustitución
Uso de la Reacción de Nicholas Intermolecular y Metátesis de Cierre de Anillos en la Formación de Éteres Cíclicos con Alto Grado de Sustitución (DEA)
Catálisis sostenible. Síntesis de azaciclos de tamaño medio (TFM)
Formación de enlaces C-C usando ácidos de Lewis. Aplicaciones de los haluros de Fe(III). (DEA)
Aplicación de las Sales de Fe (III) en la formación de enlaces C-C. Síntesis de anillos piránicos via reacción de Prins. Estudios sintéticos de éteres bioactivos de origen marino
Síntesis de oxaciclos de tamaño medio (TFM)
La ciclación de Prins y la hidrobromación regioselectiva de alquenos bajo el paradigma de la catálisis metálica sostenible
Estudios Estereoquímicos de Sacáridos en disolución
Aplicación de la ciclación de Prins en la síntesis de Tetrahidropiranos fusionados (DEA)
Desarrollo de nuevas metodologías catalizadas por sales de hierro(III) y su aplicación en la síntesis de oxa-y azaciclos
Síntesis de trans-pirrolidinas y trans-prolinas a través de hidroaminación intramolecular catalizada por sales de hierro (III). (TFM)
Cloruro de Fe(III) en la formación de enlaces C-C aplicado a la sínteis de tetrahidrofuranos (DEA)
Desarrollo y estudios mecanísticos de nuevos procesos catalizados por sales de Fe(III)
Aproximación a la síntesis de alcaloides pirrolidínicos mediante el uso de las sales de hierro (TFG)
Desarrollo de nuevas metodologías en la formación de enlaces Carbono-Carbono empleando sales de Hierro (III) y su aplicación en la síntesis de aza-ciclos (DEA)
Desarrollo de nuevas metodologías en la formación de enlaces carbono-carbono empleando sales de Hierro (III) y su aplicación en la síntesis de aza-ciclos
Aplicación de análisis bayesiano a la evaluación de cálculos químico cuánticos dedicados a la determinación estructural de productos naturales
Diseño y síntesis química de nuevos compuestos antileucémicos inspirados en Productos Naturales
Funding

Agentes terapéuticos activables por H2S para el cáncer de colon
En Ejecución

Desarrollo de nuevas metodologías para la síntesis de heterociclos de tamaño medio y su aplicación a la síntesis de productos naturales
En Ejecución

Control conformacional de plataformas moleculares para el diseño, síntesis y aplicación de jaulas orgánicas moleculares
En Ejecución

Dynamic Functional Systems for Biomedicine and Material Sciences
En Ejecución

Recyclable Polymeric Filters for Water Remediation
En Ejecución

Apoyo técnico para la elaboración de dispositivos para la liberación controlada de feromonas
En Ejecución

Acción transversal de apoyo a actividades de los proyectos relacionados con el proceso volcánico de Cumbre Vieja (La Palma)
En Ejecución

Ayuda a la Investigación del CSIC sobre ciguatera
Este proyecto tiene como objetivo desarrollar métodos de detención y seguimiento de las ciguatoxinas en alimentos, a fin de comprender sus estructuras químicas y toxicidad, mediante la aplicación…
En Ejecución

Aprovechamiento racional de los compuestos naturales mayoritarios de endemismos. CANARIASARGELIA
En Ejecución

Compuestos con potencial aplicación en el tratamiento de la Enfermedad de Huntington
Grupos de investigación del área de químicas del IPNA colaboran con GEM en la identificación de nuevos compuestos líderes con potencial actividad en el tratamiento de enfermedades raras. Los…
En Ejecución

Apoyo técnico para el seguimiento científico, la elaboración de estudios poblacionales y la realización de ensayos de detección de residuos de hexaflumuron en suelos y material vegetal en la isla de Tenerife
En Ejecución

Desarrollo de Nuevos Métodos Computacionales de Elucidación Estructural Aplicados al Estudio de los Productos Naturales Marinos
Este proyecto busca el desarrollo de nuevas herramientas de análisis basadas en el uso conjunto de la química cuántica y la espectroscopía de RMN que mejoren el proceso…
En Ejecución
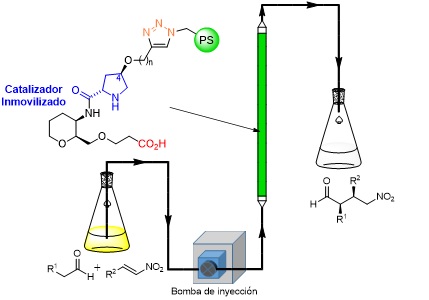
Organocatálisis asimétrica. Hacia una química más sostenible
El proyecto se centra en el desarrollo de procesos químicos que sean altamente sostenibles, utilizando para este propósito una aproximación a través de la organocatálisis asimétrica. Esta…
Finalizado

MAC-INTERREG MIMAR+ "Seguimiento, control y mitigación de proliferaciones de organismos marinos asociadas a perturbaciones humanas y cambio climático en la Región Macaronésica"
Con MIMAR+ se consolidan y expanden los avances en el conocimiento de MIMAR, pretendiendo agrupar a los agentes interesados en toda la región bajo la cobertura de un…
En Ejecución

Química Sostenible: de Moléculas Pequeñas a Sistemas Funcionales Complejos
Finalizado

Plataforma de Metabolómica y BioAnálisis. Adquisición equipo UHPLC-MS/MS (PMBA-UHPLC)
El principal objetivo del proyecto, subvencionado por la Unión Europea a través del Fondo Europeo de Desarrollo Regional (FEDER), es disponer de una plataforma metabolómica y BioAnálisis (Equipo…
Finalizado
Química sostenible: de moléculas pequeñas a sistemas funcionales complejos
Finalizado

Adquisición de Espectrómetro de Masas MALDI-TOF
El principal objetivo del proyecto, subvencionado por la Unión Europea a través del Fondo Europeo de Desarrollo Regional (FEDER), es disponer de un espectrómetro de masas, concretamente un MALDI-…
En Ejecución

Smart Multi-target Pro-drugs for Huntington (and other Neurodegenerative Diseases)
Finalizado
Síntesis de oxocenos presentes en la ciguatoxina por procesos benignos con el medioambiente
Finalizado

Valorization of a marine natural product from the Canary Islands. Preformulation and in vivo proof of concept of the norzoanthamine potential for the treatment of cartilage disorders
Finalizado

Síntesis orgánica bajo el paradigma de la sostenibilidad
Víctor S. Martín
Finalizado

Síntesis orgánica bajo el paradigma de sostenibilidad
Finalizado

Receptores Moleculares como Modelos para el Estudio de las Interacciones No Covalentes y sus Consecuencias
Finalizado

Síntesis orgánica bajo el paradigma de la sostenibilidad
Finalizado

Asignación Estereoquímica de Moléculas Orgánicas Utilizando Métodos Computacionales y RMN
Dr. Antonio Hernández Daranas
Finalizado

Cápsulas Moleculares Ensambladas por Enlaces Mecánicos: Una Nueva Aproximación a la Entrega Selectiva de Fármacos
Finalizado

Programación química de receptores Toll tipo 4: Diseño, síntesis y estudios biológicos de vacunas contra el cáncer de próstata
Los TLR constituyen un elemento clave del sistema inmunitario, ya que se expresan en células inmunitarias innatas y ayudan a reconocer los patógenos invasores. En…
Finalizado

Generación de diversidad esqueletal en lupanos como herramienta en la búsqueda de nuevos agentes anticancerosos
Finalizado

Pro-fármacos Inteligentes Auto-inmolativos como Nuevos Agentes Antitumorales
Finalizado

Investigación para el aprovechamiento de los residuos de poda agrícola de los cultivos de especies de la familia de las Proteaceae a través de la obtención de compuestos naturales con valor comercial o industrial. Optimización del cultivo
Finalizado

Investigación para el Aprovechamiento de los Residuos de Poda Agrícola de los Cultivos de Especies de la Familia de las Proteaceae a través de la Obtención de Compuestos Naturales con Valor Comercial o Industrial. Optimización del Cultivo
Finalizado

Desarrollo de Compuestos Bioactivos. Exploración de Nuevas Metodologías Sintéticas
Finalizado

Síntesis de pequeñas moléculas para cartografíar la bioactividad en el espacio químico
Finalizado

Síntesis de nuevas entidades químicas para cartografiar la bioactividad en el espacio químico
Víctor S. Martín García
Finalizado

Receptores Quirales de Cationes y Péptido-Miméticos Como Modelos para el Estudio de las Interacciones No Covalentes y sus Implicaciones
Finalizado

Evaluación de potenciales compuestos antileucémicos
Francisco Estévez Rosas
Finalizado

Valorisation of Phytochemical Natural Resources in Algeria
Annelise Lobstein
Finalizado

Aislamiento y Síntesis de Productos Naturales con Actividad Citostática de Plantas de la Familia Asteraceae Endémicas de Canarias
Jorge Triana Méndez
Finalizado

Acciones para el desarrollo y formación de personal investigador y docente en el estudio de plantas y hongos empleados en la medicina tradicional en Argelia
Juan Francisco León Oyola
Finalizado

Diseño, síntesis y evaluación citotóxica de nuevos antitumorales basados en farmacóforos de piranos y aza-ciclos
Finalizado

Desarrollo de nuevos procesos catalíticos dirigidos a la síntesis de moléculas bioactivas
Finalizado

Desarrollo de nuevos procesos catalíticos dirigidos a la síntesis de moléculas bioactivas
Víctor S. Martín García
Finalizado

Nuevos Receptores Quirales de Cationes: Diseño, Síntesis, Estudio Estructural y Aplicaciones. Aproximación al Diseño y la Síntesis de Péptido-Miméticos
Finalizado

Estudio del mecanismo de acción de potenciales compuestos antitumorales naturales y de síntesis
Francisco Estévez Rosas
Finalizado

Diseño, síntesis y evaluación citotóxica de nuevos antitumorales basados en farmacóforos de piranos y aza-ciclos. Proyectos Intramurales (CSIC)
Finalizado

Construcción eficiente de elementos estructurales privilegiados: aplicación a la síntesis de cabezas de serie biológicos y nuevos receptores moleculares
Finalizado

Diseño, Síntesis y Estudio de Nuevos Receptores Quirales de Cationes. Aplicación a la Resolución de Mezclas Racémicas y a la Catálisis Asimétrica
Finalizado

Diseño, desarrollo y aplicación de procesos dominó y multicomponente a la síntesis de nuevos antitumorales
Finalizado

Estudios estructurales y sintéticos de éteres cíclicos bioactivos de origen marino
Finalizado

Síntesis estéreoselectiva de productos naturales y análogos con actividad biológica en sus formas enantioméricas
Finalizado

Productos naturales y sintéticos con actividades anticancerígenas, anticolesterolémicas y como revertidores de multiresistencia de fármacos
Antonio González González
Finalizado
People
Juan Ignacio Padrón Peña
Tomás Martín Ruíz
Romen Carrillo Fumero
Jimena Scoccia
Lidia A. Pérez-Márquez
Yaiza Pérez
Antonio Hernández Daranas
Tanausú Santos
David Santana
Manuel Rondelli
Cristina Cuadrado García
Víctor Manuel de la Iglesia Rial
Ezequiel Santiago De Quintana Morales
David Tejedor Aragón
Raquel Diana Rivero
María Pilar González Figueras
Ignacio Brouard Martín
Pedro Antonio de Armas González
Irma García Monzón
Samuel Delgado Hernández
María Víctoria Sinka
Daniel Alejandro Cruz Perdomo
Davide Andrea Coppini
Humberto Adrián Rodríguez Hernández
Publications
Evaluation of Oxasqualenoids from the Red Alga Laurencia viridis against Acanthamoeba
Acanthamoeba genus is a widely distributed and opportunistic parasite with increasing importance worldwide as an emerging pathogen in the past decades. This protozoan has an active trophozoite stage, a cyst stage, and is dormant and very resistant. It can cause Acanthamoeba keratitis, an ocular sight-threatening disease, and granulomatous amoebic encephalitis, a chronic, very fatal brain pathology. In this study, the amoebicidal activity of sixteen Laurencia oxasqualenoid metabolites and semisynthetic derivatives were tested against Acanthamoeba castellanii Neff. The results obtained point out that iubol (3) and dehydrothyrsiferol (1) possess potent activities, with IC values of 5.30 and 12.83 µM, respectively. The hydroxylated congeners thyrsiferol (2) and 22-hydroxydehydrothyrsiferol (4), active in the same value range at IC 13.97 and 17.00 µM, are not toxic against murine macrophages; thus, they are solid candidates for the development of new amoebicidal therapies.
Lorenzo-Morales, Jacob; Díaz-Marrero, Ana R. ; Cen-Pacheco, Francisco; Sifaoui, Inés; Reyes-Batlle, María; Souto, María L.; Hernández Daranas, Antonio; Piñero, José E.; Fernández, José J.
Antiprotozoal activities of marine polyether triterpenoids
Chagas disease and leishmaniasis are tropical neglected diseases caused by kinetoplastids protozoan parasites of Trypanosoma and Leishmania genera, and a public health burden with high morbidity and mortality rates in developing countries. Among difficulties with their epidemiological control, a major problem is their limited and toxic treatments to attend the affected populations; therefore, new therapies are needed in order to find new active molecules. In this work, sixteen Laurencia oxasqualenoid metabolites, natural compounds 1–11 and semisynthetic derivatives 12–16, were tested against Leishmania amazonensis, Leishmania donovani and Trypanosoma cruzi. The results obtained point out that eight substances possess potent activities, with IC values in the range of 5.40–46.45 µM. The antikinetoplastid action mode of the main metabolite dehydrothyrsiferol (1) was developed, also supported by AFM images. The semi-synthetic active compound 28-iodosaiyacenol B (15) showed an IC 5.40 µM against Leishmania amazonensis, turned to be non-toxic against the murine macrophage cell line J774A.1 (CC > 100). These values are comparable with the reference compound miltefosine IC 6.48 ± 0.24 and CC 72.19 ± 3.06 μM, suggesting that this substance could be scaffold for development of new antikinetoplastid drugs.
Díaz-Marrero, Ana R. ; López-Arencibia, Atteneri; Bethencout-Estrella, Carlos J.; Cen-Pacheco, Francisco; Sifaoui, Ines; Hernández Creus, Alberto; Duque-Ramírez, María Clara; Souto, María L.; Hernández Daranas, Antonio ; Lorenzo-Morales, Jacob; Piñero, José E.; Fernández, José J.
Centaurea microcarpa Coss. & Dur. (Asteraceae) extracts: New cyanogenic glucoside and other constituents
The phytochemical investigation of both chloroform and ethyl acetate extracts of Centaurea microcarpa Coss. & Dur. led to the isolation of a new cyanogenic glucoside 6'-methacrylate prunasin (3) together with seven known compounds: hydroxy-11β,13-dihydro onopordaldehyde (1), β-sitosterol (2), daucosterol (4), nepetin (5), prunasin (6), astragalin (7) and 7-O-β-D-glucopyranosyl centaureidin (8). Their structures were established by spectral analysis, mainly UV, IR, ESI-MS, 1D & 2D-NMR experiments (COSY, HSQC, HMBC and ROESY).
Baatouche, Samia; Cheriet, Thamere; Sarri, Djamel; Mekkiou, Ratiba; Boumaza, Ouahiba; Benayache, Samir; Benayache, Fadila; Brouard, Ignacio; León, Francisco ; Seghiri, Ramdane
Recent Advances in the Synthesis of 2H-Pyrans
In this review, we discuss the nature of the different physicochemical factors affecting the valence isomerism between 2H-pyrans (2HPs) and 1-oxatrienes, and we describe the most versatile synthetic methods reported in recent literature to access to 2HPs, with the only exception of 2HPs fused to aromatic rings (i.e., 2H-chromenes), which are not included in this review.
Tejedor, David; Delgado-Hernández, Samuel; Diana-Rivero, Raquel; Díaz-Díaz, Abián; García-Tellado, Fernando
A Focused Library of NO-Donor Compounds with Potent Antiproliferative Activity Based on Green Multicomponent Reactions
Cancer is the second leading cause of death worldwide. Herein, a strategy to quickly and efficiently identify novel lead compounds to develop anticancer agents, using green multicomponent reactions followed by antiproliferative activity and structure–activity relationship studies, is described. A second-generation focused library of nitric oxide-releasing compounds was prepared by microwave-assisted Passerini and Ugi reactions. Nearly all compounds displayed potent antiproliferative activities against a panel of human solid tumor cell lines, with 1-phenyl-1-[(tert-butylamino)carbonyl]methyl 3-[(3-phenylsulfonyl-[1,2,5]oxadiazol-4-yl N-oxide)oxy]benzoate (4 k) and N-[1-(tert-butylaminocarbonyl)-1-phenylmethyl]-N-(4-methylphenyl)-3-(3-phenylsulfonyl-[1,2,5]oxadiazol-4-yl N-oxide)oxyphenyl carboxamide (6 d) exhibiting the strongest activity on SW1573 lung cell line (GI=110 and 21 nm) with selectivity indices of 70 and 470, respectively. Preliminary mechanistic studies suggest a relationship between NO release and antiproliferative activity. Our strategy allowed the rapid identification of at least two molecules as future candidates for the development of potent antitumor drugs
Ingold, Marina; Colella, Lucía; Hernández, Paola; Batthyány, Carlos; Tejedor, David; Puerta, Adrian; García-Tellado, Fernando; Padrón, José M.; Porcal, Williams; López, Gloria V.
Combining the Power of J Coupling and DP4 Analysis on Stereochemical Assignments: The J-DP4 Methods
A systematic study to include J couplings into DP4 formalism (J-DP4) led to the development of three alternative strategies. The dJ-DP4 (direct) approach involves a new DP4-like equation including an additional probability term given by J. The iJ-DP4 (indirect) approach explores the original DP4 method with a restricted conformational search. Despite both strategies performing better than DP4, their combined use (iJ/dJ-DP4) provided the best results, with a 2.5-fold performance improvement at similar or lower computational cost.
Grimblat, Nicolás; Gavín, José A.; Hernández Daranas, Antonio; Sarotti, Ariel M.
Collaborations
Romen Carrillo Fumero

Contact information
Antonio Hernández Daranas

Contact information
News/Blog
- 25 November 2022
- 31 May 2022
- 19 May 2022
- 17 September 2021
- 14 October 2021
- 23 June 2021
Other research groups