Anthropogenic Perturbations to the Atmospheric Molybdenum Cycle
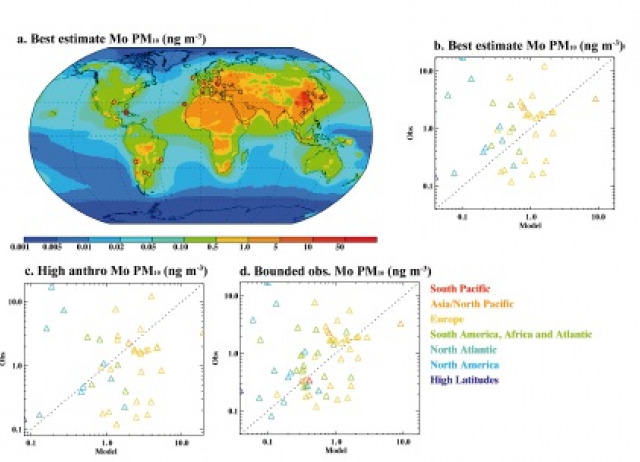
Molybdenum (Mo) is a key cofactor in enzymes used for nitrogen (N) fixation and nitrate reduction, and the low availability of Mo can constrain N inputs, affecting ecosystem productivity. Natural atmospheric Mo aerosolization and deposition from sources such as desert dust, sea‐salt spray, and volcanoes can affect ecosystem function across long timescales, but anthropogenic activities such as combustion, motor vehicles, and agricultural dust have accelerated the natural Mo cycle. Here we combined a synthesis of global atmospheric concentration observations and modeling to identify and estimate anthropogenic sources of atmospheric Mo. To project the impact of atmospheric Mo on terrestrial ecosystems, we synthesized soil Mo data and estimated the global distribution of soil Mo using two approaches to calculate turnover times. We estimated global emissions of atmospheric Mo in aerosols (<10 μm in diameter) to be 23 Gg Mo yr‐1, with 40 to 75% from anthropogenic sources. We approximated that for the top meter of soil, Mo turnover times range between 1,000 to 1,000,000 years. In some industrialized regions, anthropogenic inputs have enhanced Mo deposition 100‐fold, lowering the soil Mo turnover time considerably. Our synthesis of global observational data, modeling, and a mass balance comparison with riverine Mo exports suggest that anthropogenic activity has greatly accelerated the Mo cycle, with potential to influence N‐limited ecosystems.
Wong, Michelle Y.; Rathod, Sagar D.; Marino, Roxanne; Li, Longlei; Howarth, Robert W.; Alastuey, Andres; Alaimo, Maria Grazia; Barraza, Francisco; Carneiro, Manuel Castro; Chellam, Shankararaman; Chen Yu-Cheng; Cohen, David D.; Connelly, David; Dongarra, Gaetano; Gomez, Dario; Hand, Jenny; Harrison, R.M.; Hopke, Philip K.; Hueglin, Christoph; Kuang, Yuan-wen; Lambert, Fabrice; Liang, James; Losno, Remi; Maenhaut, Willy; Milando, Chad; Couto Monteiro, Maria Inês; Morera-Gómez, Yasser; Querol, Xavier; Rodríguez, Sergio; Smichowski, Patricia; Varrica, Daniela; Xiao, Yi-hua; Xu, Yangjunjie; Mahowald, Natalie M.
Validated removal of nuclear pseudogenes and sequencing artefacts from mitochondrial metabarcode data
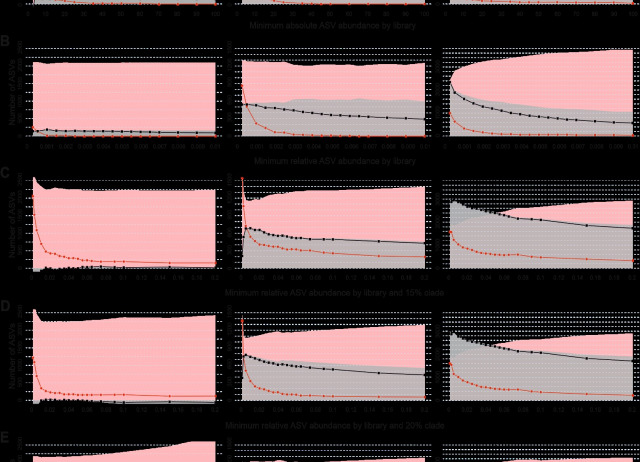
Metabarcoding of Metazoa using mitochondrial genes may be confounded by both the accumulation of PCR and sequencing artefacts and the co-amplification of nuclear mitochondrial pseudogenes (NUMTs). The application of read abundance thresholds and denoising methods is efficient in reducing noise accompanying authentic mitochondrial amplicon sequence variants (ASVs). However, these procedures do not fully account for the complex nature of concomitant sequences and the highly variable DNA contribution of specimens in a metabarcoding sample. We propose, as a complement to denoising, the metabarcoding Multidimensional Abundance Threshold Evaluation (metaMATE) framework, a novel approach that allows comprehensive examination of multiple dimensions of abundance filtering and the evaluation of the prevalence of unwanted concomitant sequences in denoised metabarcoding datasets. metaMATE requires a denoised set of ASVs as input, and designates a subset of ASVs as being either authentic (mitochondrial DNA haplotypes) or nonauthentic ASVs (NUMTs and erroneous sequences) by comparison to external reference data and by analysing nucleotide substitution patterns. metaMATE (i) facilitates the application of read abundance filtering strategies, which are structured with regard to sequence library and phylogeny and applied for a range of increasing abundance threshold values, and (ii) evaluates their performance by quantifying the prevalence of nonauthentic ASVs and the collateral effects on the removal of authentic ASVs. The output from metaMATE facilitates decision-making about required filtering stringency and can be used to improve the reliability of intraspecific genetic information derived from metabarcode data. The framework is implemented in the metaMATE software (available at https://github.com/tjcreedy/metamate).
Andújar, Carmelo; Creedy, Thomas J.; Arribas, Paula; López, Heriberto; Salces-Castellano, Antonia; Pérez‐Delgado, Antonio José; Vogler, Alfried P.; Emerson, Brent C.
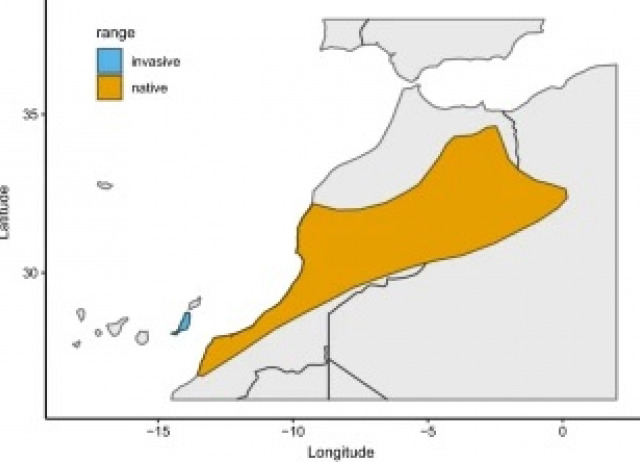
Exploring the role of life history traits and introduction effort in understanding invasion success in mammals: a case study of Barbary ground squirrels
Invasive species–species that have successfully overcome the barriers of transport, introduction, establishment, and spread—are a risk to biodiversity and ecosystem function. Introduction effort is one of the main factors underlying invasion success, but life history traits are also important as they influence population growth. In this contribution, we first investigated life history traits of the Barbary ground squirrel, Atlantoxerus getulus, a species with a very low introduction effort. We then studied if their invasion success was due to a very fast life history profile by comparing their life history traits to those of other successful invasive mammals. Next, we examined whether the number of founders and/or a fast life history influences the invasion success of squirrels. Barbary ground squirrels were on the fast end of the “fast-slow continuum”, but their life history was not the only contributing factor to their invasion success, as the life history profile is comparable to other invasive species that do not have such a low introduction effort. We also found that neither life history traits nor the number of founders explained the invasion success of introduced squirrels in general. These results contradict the concept that introduction effort is the main factor explaining invasion success, especially in squirrels. Instead, we argue that invasion success can be influenced by multiple aspects of the new habitat or the biology of the introduced species.
van der Marel, Annemarie; Waterman, Jane M.; López-Darias, Marta
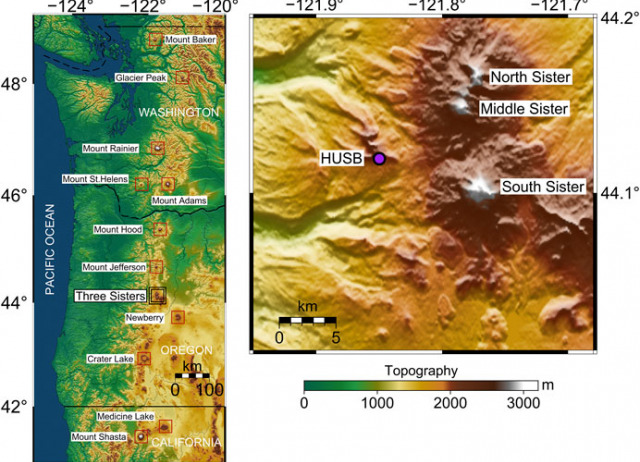
Time-Scales of Inter-eruptive Volcano Uplift Signals: Three Sisters Volcanic center, Oregon (USA)
A classical inflation-eruption-deflation cycle of a volcano is useful to conceptualize the time-evolving deformation of volcanic systems. Such a model predicts accelerated uplift during pre-eruptive periods, followed by subsidence during the co-eruptive stage. Some volcanoes show puzzling persistent uplift signals with minor or no other geophysical or geochemical variations, which are difficult to interpret. Such temporal behaviors are usually observed in large calderas (e.g., Yellowstone, Long Valley, Campi Flegrei, Rabaul), but less commonly for stratovolcanoes. Volcano deformation needs to be better understood during inter-eruptive stages, to assess its value as a tool for forecasting eruptions and to understand the processes governing the unrest behavior. Here, we analyze inter-eruptive uplift signals at Three Sisters, a complex stratovolcano in Oregon (United States), which in recent decades shows persistent inter-eruptive uplift signals without associated eruptive activity. Using a Bayesian inversion method, we re-assessed the source characteristics (magmatic system geometry and location) and its uncertainties. Furthermore, we evaluate the most recent evolution of the surface deformation signals combining both GPS and InSAR data through a new non-subjective linear regularization inversion procedure to estimate the 26 years-long time-series. Our results constrain the onset of the Three Sisters volcano inflation to be between October 1998 and August 1999. In the absence of new magmatic inputs, we estimate a continuous uplift signal, at diminishing but detectable rates, to last for few decades. The observed uplift decay observed at Three Sisters is consistent with a viscoelastic response of the crust, with viscosity of ∼1018 Pa s around a magmatic source with a pressure change which increases in finite time to a constant value. Finally, we compare Three Sisters volcano time series with historical uplift at different volcanic systems. Proper modeling of scaled inflation time series indicates a unique and well-defined exponential decay in temporal behavior. Such evidence supports that this common temporal evolution of uplift rates could be a potential indicator of a rather reduced set of physical processes behind inter-eruptive uplift signals.
Rodríguez-Molina, Sara; González, Pablo J.; Charco, María; Negredo, Ana M.; Schmidt, David A.
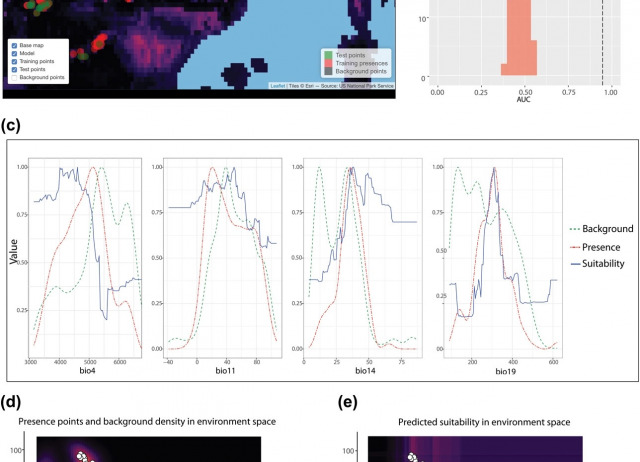
ENMTools 1.0: an R package for comparative ecological biogeography
The ENMTools software package was introduced in 2008 as a platform for making measurements on environmental niche models (ENMs, frequently referred to as species distribution models or SDMs), and for using those measurements in the context of newly developed Monte Carlo tests to evaluate hypotheses regarding niche evolution. Additional functionality was later added for model selection and simulation from ENMs, and the software package has been quite widely used. ENMTools was initially implemented as a Perl script, which was also compiled into an executable file for various platforms. However, the package had a number of significant limitations; it was only designed to fit models using Maxent, it relied on a specific Perl distribution to function, and its internal structure made it difficult to maintain and expand. Subsequently, the R programming language became the platform of choice for most ENM studies, making ENMTools less usable for many practitioners. Here we introduce a new R version of ENMTools that implements much of the functionality of its predecessor as well as numerous additions that simplify the construction, comparison and evaluation of niche models. These additions include new metrics for model fit, methods of measuring ENM overlap, and methods for testing evolutionary hypotheses. The new version of ENMTools is also designed to work within the expanding universe of R tools for ecological biogeography, and as such includes greatly simplified interfaces for analyses from several other R packages.
Warren, Dan L.; Matzke, Nicholas J.; Cardillo, Marcel; Baumgartner, John B.; Beaumont, Linda J.; Turelli, Michael; Glor, Richard E.; Huron, Nicholas A.; Simões, Marianna; Iglesias, Teresa L.; Piquet, Julien C.; Dinnage, Russell
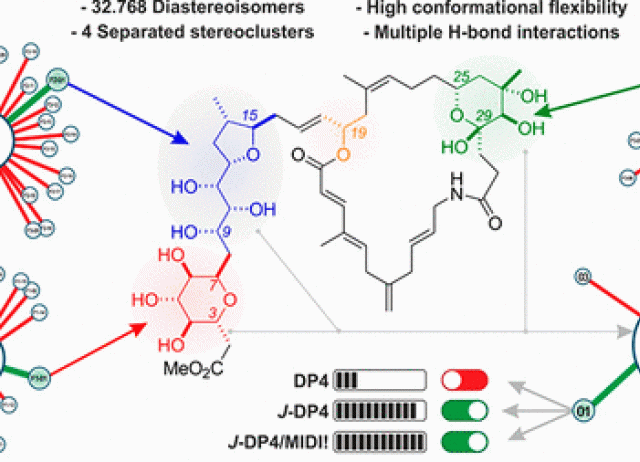
Are Computational Methods Useful for Structure Elucidation of Large and Flexible Molecules? Belizentrin as a Case Study
Quantum mechanical NMR methods are progressively becoming decisive in structure elucidation. However, problems arise using low-level calculations for complex molecules, whereas methods using higher levels of theory are not practical for large molecules. This report outlines a synergistic effort employing computationally inexpensive quantum mechanical NMR calculations with conformer selection incorporating 3JHH values as a way to solve the structure of large, complex, and highly flexible molecules using readily available computational resources with belizentrin as a case study.
Hernández Daranas, Antonio; Sarotti, Ariel M.
The role of the brown bear Ursus arctos as a legitimate megafaunal seed disperser
Megafaunal frugivores can consume large amounts of fruits whose seeds may be dispersed over long distances, thus, affecting plant regeneration processes and ecosystem functioning. We investigated the role of brown bears (Ursus arctos) as legitimate megafaunal seed dispersers. We assessed the quantity component of seed dispersal by brown bears across its entire distribution based on information about both the relative frequency of occurrence and species composition of fleshy fruits in the diet of brown bears extracted from the literature. We assessed the quality component of seed dispersal based on germination experiments for 11 fleshy-fruited plant species common in temperate and boreal regions and frequently eaten by brown bears. Across its distribution, fleshy fruits, on average, represented 24% of the bear food items and 26% of the total volume consumed. Brown bears consumed seeds from at least 101 fleshy-fruited plant species belonging to 24 families and 42 genera, of which Rubus (Rosaceae) and Vaccinium (Ericaceae) were most commonly eaten. Brown bears inhabiting Mediterranean forests relied the most on fleshy fruits and consumed the largest number of species per study area. Seeds ingested by bears germinated at higher percentages than those from whole fruits, and at similar percentages than manually depulped seeds. We conclude that brown bears are legitimate seed dispersers as they consume large quantities of seeds that remain viable after gut passage. The decline of these megafaunal frugivores may compromise seed dispersal services and plant regeneration processes.
García-Rodríguez, Alberto; Albrecht; Jörg, Szczutkowska, Sylwia; Valido, Alfredo; Farwing, Nina; Selva, Nuria
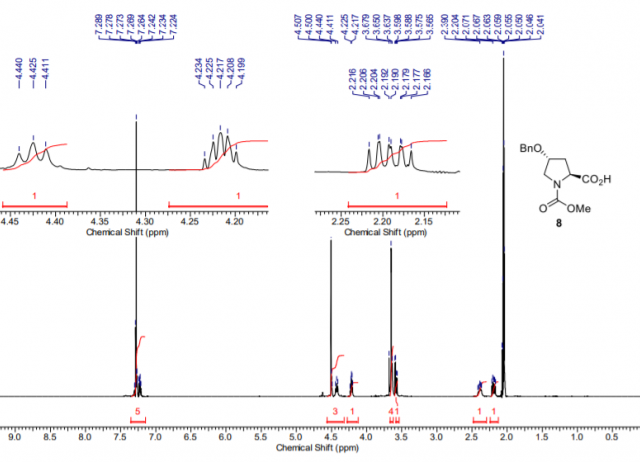
“Doubly Customizable” Unit for the Generation of Structural Diversity: From Pure Enantiomeric Amines to Peptide Derivatives
Readily available, low-cost 4R-hydroxy-l-proline (Hyp) is introduced as a “doubly customizable” unit for the generation of libraries of structurally diverse compounds. Hyp can be cleaved at two points, followed by the introduction of new functionalities. In the first cycle, the removal and replacement of the carboxylic group are carried out, followed (second cycle) by the scission of the 4,5-position and manipulation of the resulting chains. In this way, three new chains are generated and can be transformed independently to afford a diversity of products with tailored substituents, such as β-amino aldehydes, diamines, β-amino acid derivatives, including N-alkylated ones, or modified peptides. Many of these products are high-profit compounds but, in spite of their commercial value, are still scarce. Moreover, the process takes place with stereochemical control, and either pure R or S isomers can be obtained with small variations of the synthetic route.
Hernández, Dácil; Carro, Carmen; Boto, Alicia
Application of stimuli-responsive materials for extraction purposes
Stimuli-responsive materials, frequently designated as “smart/intelligent materials”, can modify their structure or properties by either a biological, physical, or chemical stimulus which, if properly controlled, could be used for specific applications. Such materials have been studied and exploited in several fields, like electronics, photonics, controlled drugs administration, imaging and medical diagnosis, among others, as well as in Analytical Chemistry where they have been used as chromatographic stationary phases, as part of sensors and for extraction purposes. This review article pretends to provide an overview of the most recent applications of these materials (mostly polymeric materials) in sample preparation for extraction purposes, as well as to provide a general vision of the current state-of-the-art of this field, their potential use and future applications.
González-Sálamo, Javier; Ortega-Zamora, Cecilia; Carrillo Fumero, Romen; Hernández-Borges, Javier
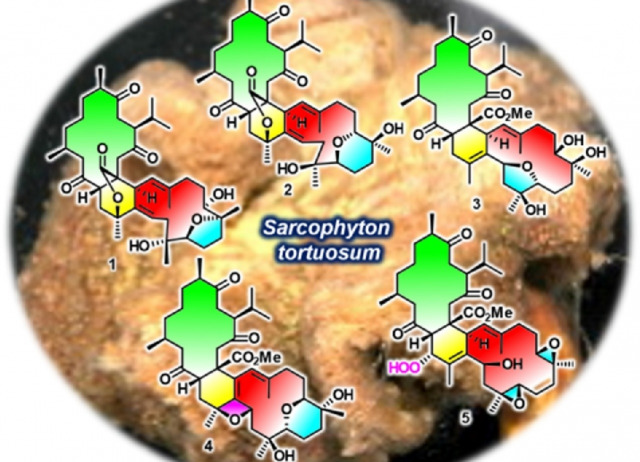
Polyoxygenated anti-inflammatory biscembranoids from the soft coral Sarcophyton tortuosum and their stereochemistry
Five novel biscembranoids, ximaolides H–L (1–5), along with four known related compounds (6–9) were isolated from the Hainan soft coral Sarcophyton tortuosum. The structures of the new compounds were determined by extensive spectroscopic analysis, quantum chemical calculations, and/or by comparing their CD spectra with those of the known compounds. Compounds 1 and 2 are the first examples of biscembranoids bearing a 1, 35-bridged lactone moiety, 4 is the first biscembranoid comprising an uncommon oxetane ring, and 5 represents the first 36-peroxyl biscembranoid. Ximaolides I (2), K (4) and F (9) exhibited interesting anti-inflammatory activity by the inhibition of LPS-induced TNF-α protein release in RAW264.7 macrophages.
Li, Yufen; Li, Songwei; Cuadrado, Cristina; Gao, Chenglong; Wu, Qihao; Li, Xiaolu; Pang, Tao; Hernández Daranas, Antonio; Guo, Yuewei; Li, Xuwen